Resonance structures and stability
- Benjamin Hui
- Mar 3, 2019
- 5 min read
Electrons are highly energetic particles. The more there are in a molecule, the higher energy that molecule would have overall. Should we go so far to say that the molecule would therefore be unstable though? For neutral molecules (which most are), them having more electrons usually translates to having more atoms i.e. the molecule is larger. Take the Taxol molecule for example, a potent anticancer drug. This complicated molecule is by all standards pretty large, and has many electrons. But by its very nature of being large with more atoms, the electron cloud is spread out over more nuclei, thereby offsetting the higher energy. Taxol is therefore stable i.e. it won't react with oxygen in the air nor will it spontaneously decompose if left to stand.

A different story arises when we talk about charged (either positive or negative) molecules. We know these are unstable and it is almost impossible to isolate one in the lab. They have an excess charge to atom ratio. In organic chemistry, most positively-charged molecules encountered are carbocations, and negatively-charged ones are more diverse such as carbanions, alkoxides, thiolates, carboxylates etc. Regardless, when they are generated in a reaction, the first thing they would do is to try to alleviate the charge, either by accepting or giving away electrons, or to spread the charge over more atoms in a process known as resonance.
Resonance can only occur if the charge is adjacent to an atom that has a multiple bond (either double or triple). A good example would be the carboxylate anion:

Starting from the left side anion, the negative charge that's on an oxygen atom is adjacent to a double bond. The lone pair of electrons responsible for this charge can then move to form a new double bond, while the old one is broken and its constituent electrons move into the other oxygen as a lone pair, giving it a negative formal charge. The right side anion can become the left side one again by reversal of the electron flow. These two resonance forms shuttle back and forth rapidly, therefore we use a double-headed arrow to represent this. The average result (called the resonance hybrid) is the delocalization of the negative charge between the two oxygen atoms, giving each an effective charge of -1/2. We say that the carboxylate anion has two resonance structures.
Here is a less obvious example, a protonated carbonyl group, This is usually the first step of any reaction involving a carbonyl group: it has to be activated by an acid catalyst. Does this have resonance structures? Let's have a look:

We start off where the positive charge is, which is on the oxygen. Does it have any adjacent atoms that have multiple bonds? We see that the neighbouring carbon does have a double bond, and the oxygen itself is part of that bond. This double bond can break, with the electrons moving into the oxygen to relieve its positive charge. Concurrently, the carbon atom gains a positive charge because it has lost its own electron that was in the bond. So, activated (protonated) carbonyls do have resonance structures. The resonance hybrid is represented by a dotted line where the double bond is, showing that it is transient.
These resonance structures can also explain why nucleophiles always attack the carbon atom of the carbonyl group, and never the oxygen. If the nucleophile attacks the oxygen, there would be 10 electrons around oxygen in the transition state - a violation of the octet rule.

Yet another example of resonance involving positive charges is the allyl cation:

The cation is generated from the corresponding allyl halide. This might look familiar because it's the first step of an Sn1 substitution. Applying what we've learned, we check for multiple bonds adjacent to the charge and find a double bond. This bond can break, and its electrons move across to form a new double bond, relieving the positive charge but at the same time generating another at the other end. The resonance hybrid is then represented by a dotted semicircle with a + sign, showing that the positive charge is delocalized between the two ends of the molecule.
All the examples we have seen thus far have two resonance structures. The more resonance structures a molecule has, the more stable it is. This applies to both charged and neutral molecules. Let's look at a couple of examples. These are phenolates, the conjugate bases of phenols.

At first glance, there's a lot of conjugation going on in these molecules, but we shouldn't get intimidated by that. As long as we work steadily, following the electrons around step-by-step, this problem can be solved easily. Let's look at the left phenolate, and we'll start off where the negative charge is, at the oxygen. Any adjacent multiple bonds? Yes there are, so we can start moving electrons around starting with a.

It is very important to ensure that those atoms hosting the lone pair as it is shuttling across the molecule receive a negative charge! Also, don't forget the forming and breaking of double bonds in the process. When we get to c, there are two different paths the charge can take: It can continue within the same ring to give g (red arrows), or it can enter the second ring to give d. Both of these are unique resonance structures and so are counted. Therefore, be on the lookout for these forked paths when you analyze other molecules. This particular molecule therefore has a total of 7 resonance structures (we also count the starting structure).
The other molecule is more interesting and will test your electron pushing skills. Starting from 1, we move out in the same manner as before.
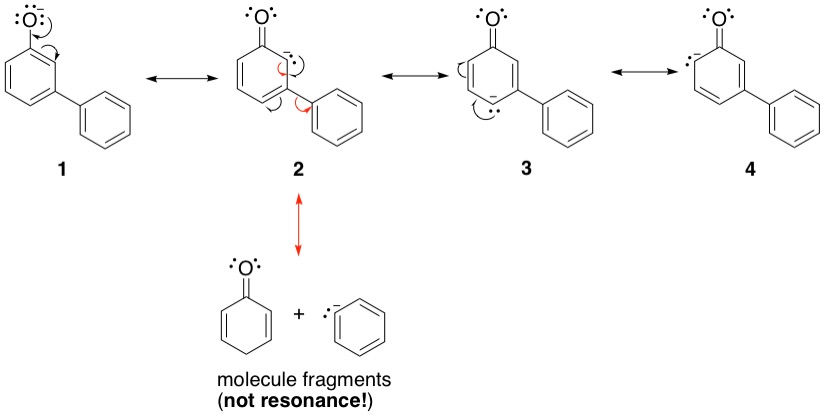
This time, when we get to the junction between the two rings, we find that the lone pair is now unable to delocalize into the second ring without breaking the bond connecting the two rings. Resonance does not count when a molecule fragments. Therefore, this example only has 4 resonance structures. Phenolate a therefore is the more stable one.
Relationship between resonance structures and acidity:
During our organic chemistry journey, we encounter lots of organic acids and will face problems asking us to predict and compare acidities i.e. pKa values. Remember the ARIO mnemonic? R refers to resonance structures, and should be what we look for after determining there is no aromaticity (A) gain or loss. The general rule is that the more resonance structures an acid's conjugate base has, the more acidic (lower pKa) it is. This makes sense because more resonance structures makes the conjugate base more stable, and it therefore wants to remain that way, with its proton gone. This is demonstrated in the phenolate examples we just looked at above.

The conjugate acid of a, 4-phenylphenol has a pKa of 9.5, 0.2 units lower than 3-phenylphenol (pKa = 9.7). This means it is twice as acidic (1 pKa unit = 10x difference, therefore 0.2 units = 2x difference). And this arises from a having 3 more resonance structures than 1.
For practice, try out the problem below. It is similar in concept to these examples we've seen, but objective is to practice electron pushing and drawing resonance structures. For reference, the NO2 group is a nitro group so look it up if you're not familiar with its structure.

Click here for the solutions.



Comments